|
|
 |
 |
 |
| E1.2
HOW WE CALCULATE DENSITY |
 |
 |
LINKS |
 |
|
 |
 |
 |
Each substance has its own density.
For example, for copper,
the mass is 71.4 grams |
 |
 |
|
 |
| |
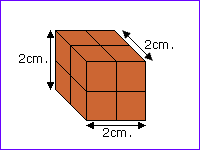 |
and the volume is 8 cm³
So the density = 71.4 / 8 =
8.9 g per cm³.
If the lump of copper had been twice the volume it would
be twice the mass. The density comes out the same. |
| |
|
|
| |
|
|
|
|
 |
|
| a
Science Enhancement Programme CD-ROM 2005 |
|
user
guide |
| |
|
|
|
|