|
|
 |
 |
 |
| W1.3
WHAT IS AN ACID? |
 |
 |
LINKS |
 |
|
 |
 |
 |
Some
acids
Acids have molecular structures. Here are some examples:
ethanoic acid
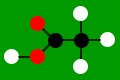
nitric acid

|
 |
sulfuric
acid

hydrochloric acid

Note: we have met this last substance
before as hydrogen chloride. When dissolved in water
it is called hydrochloric acid. |
|
|
|
 |
|
| a
Science Enhancement Programme CD-ROM 2005 |
|
user
guide |
| |
|
|
|
|