|
|
 |
 |
 |
| L2.1
STRUCTURE AND MELTING POINT |
 |
 |
LINKS |
 |
|
 |
 |
 |
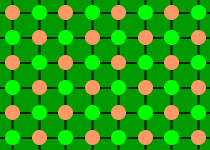
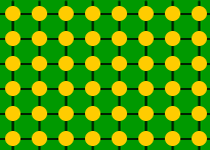
Salt
and lead are examples of giant structures:
| Structure of salt |
 |
| Structure of lead |
 |
|
 |
With each atom held in place by bonds to its neighbours, salt
and lead have high melting points.
The bonds between atoms in salt are stronger than those in
lead, which is why salt has a higher melting point.
In general, giant structures have high melting points,
well above room temperature.
|
|
|
 |
|
| a
Science Enhancement Programme CD-ROM 2005 |
|
user
guide |
| |
|
|
|
|