Notes on the video
Students should notice the following changes:
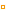 |
There is bubbling,
and white solid appears in the test tube, as soon as
the calcium slides into the water. |
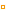 |
The bag inflates. |
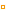 |
After a few hours, the white
solid seems to be a dry powder. |
The bag inflating is evidence that at least one reaction
product is in the gas state.
The test tube was left for a few hours to allow the white
powder to dry out. (Students can see that, earlier in the
video, the white solid was wet, with a small amount of water:
this is just water which was not needed for the reaction
and it is not a reaction product).
|